Exercise
Exercise has enormous health benefits. One of its major benefits is to improve insulin sensitivity in skeletal muscle thus providing protection against metabolic disease. Despite many decades of research, the mechanism for this effect is not known.
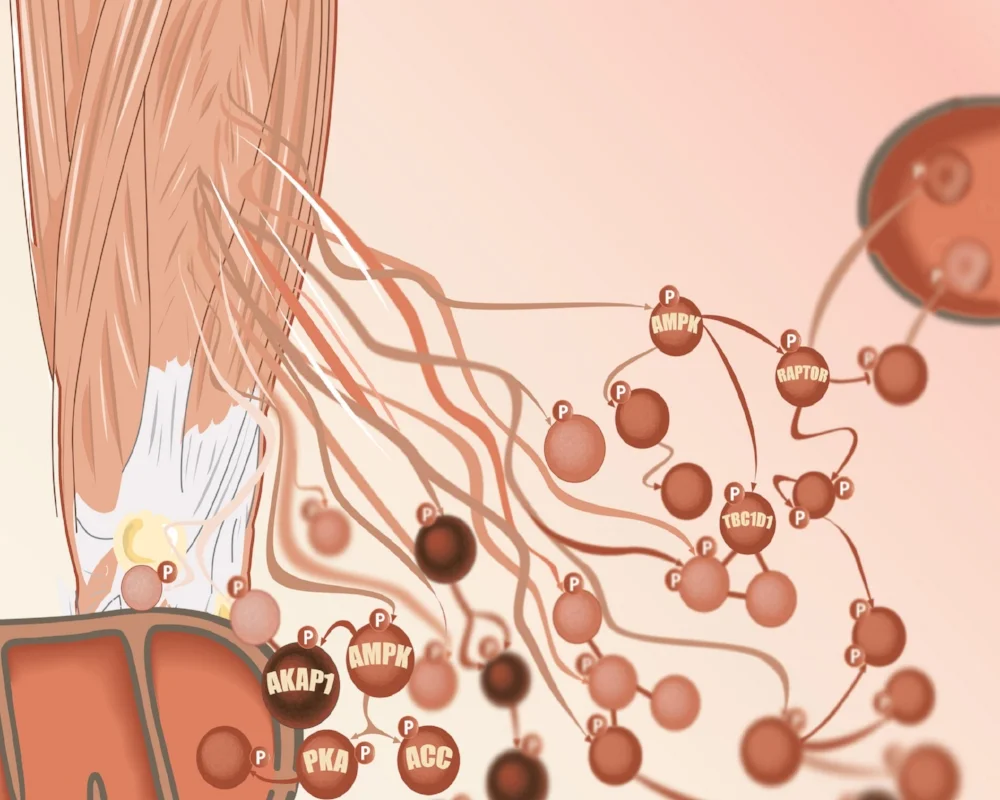
In collaboration with Jørgen Wojtaszewski from the University of Copenhagen we have embarked upon an ambitious program in humans to investigate the mechanism by which exercise improves muscle insulin sensitivity. This involves the use of Copenhagen’s novel one-legged exercise model combined with muscle biopsies and accurate measures of muscle insulin sensitivity. These biopsies were analysed by Sean Humphrey’s state of the art phosphoproteomic pipeline and data was analysed by Elise Needham, an incredibly talented PhD student in our group. This has led to spectacular novel insights into muscle insulin sensitivity involving cross talk between the mTOR and AMPK signaling pathways.
We measured muscle biopsy phosphoproteomes to determine how exercise rewires insulin signalling. We found that exercise potentiates insulin signalling, as well as causing divergent and emergent phosphorylation events. A small subset of these phosphosites are shown below and the data are available to explore at www.personalisedphospho.com. The vast majority of phosphorylation sites haven’t been characterised in insulin signalling before. There are many opportunities to expand our knowledge of how insulin regulates cellular processes.
Out of the thousands of phosphosites regulated by exercise and insulin, it is difficult to determine which of these are most important to the biological outcomes of interest, such as muscle glucose uptake. We developed a method called “personalised phosphoproteomics” to tackle this problem. Personalised phosphoproteomics takes advantage of the observation that even amongst healthy subjects, there is a remarkably broad range in the biological response to exercise. We have recently discovered that this ‘uniqueness’ is also mirrored right down to the molecular level, with humans having their own phosphoproteome fingerprint that governs their response to exercise.
Applying personalised phosphoproteomics by associating leg-specific glucose uptake measures with the phosphoproteome profiles prioritised 129 phosphosites of interest out of the >2,000 we found to be regulated using discrete comparisons. This smaller set of phosphosites were enriched for those with a reported function, and for biological relevance to glucose uptake. This included unexpected communication between the kinases mTORC1 and AMPK, involving phosphorylation on S377 of AMPK by mTORC1, for which we found a role in metabolic regulation.
Personalised phosphoproteomics can be applied to a range of contexts in diverse cohorts, to improve our understanding of how health outcomes vary dynamically across the population.
Studies are currently underway using similar approaches in individuals with insulin resistance or type 2 diabetes. Stay tuned.
This figure represents the phosphoproteome of one individual. Each box represents a phosphosite, and the colour represents how different that phosphosite is to the average levels of that phosphosite in the whole group of individuals we studied.