September 2025
Muscle very long-chain ceramides associate with insulin resistance independently of obesity
The role of muscle lipids in insulin resistance has been hotly debated, with ceramides and diacylglycerols (DAGs) often in the spotlight. In our new study using genetically diverse mice, we find that most muscle lipids track with obesity rather than insulin resistance itself. Strikingly, only very long-chain ceramides consistently associate with systemic insulin resistance. Our findings help cut through the controversy and points towards certain ceramides species, not DAGs, as key players in metabolic disease.
Genetic variance in the murine defensin locus modulates glucose homeostasis
Insulin resistance and the gut microbiome have been linked to almost every chronic disease. Here, we uncover gut-derived antimicrobial defensin peptides as a key driver of both. Through genetic mapping of systemic insulin sensitivity and subsequent systems biology investigative approaches, we identify alpha-defensin 26 (Defa26) as the likely causal gene in this region and observed higher relative abundance of metabolically beneficial microbes such as Akkermansia muciniphila. We validated these association by synthesising Defa26 and supplementing the diets of two different mouse strains. In C57BL6/J mice, Defa26 improved insulin sensitivity and increased A. muciniphila abundance. In contrast, A/J mice fed Defa26 exhibited beta-cell dysfunction, hypoinsulinemia, glucose intolerance and muscle wasting. Our findings have important implications for precision medicine and provide a mechanistic link between genetics, the gut microbiome, and metabolic health
Read full article here
***
August 2025
ADS-Pincus Taft Young Investigator Award
Congratulation to Aaron Lambert on being awarded the ADS-Pincus Taft Young Investigator Award at the Australian Diabetes Congress which was held on the Gold Coast from 20-222 August.
The ADS-Pincus Taft Young Investigator Award is to the value of $2000. The finalists will be categorised into Discovery Science and the award will be given to the best oral presentation by a junior ADS member at the Australasian Diabetes Congress as judged by peer review and a committee appointed by the ADS Council.
The insulin signalling network
Insulin signalling is a central regulator of metabolism, orchestrating nutrient homeostasis and coordinating carbohydrate, protein and lipid metabolism. This network operates through dynamic, tightly regulated protein phosphorylation events involving key kinases such as AKT, shaping cellular responses with remarkable precision. Advances in phosphoproteomics have expanded our understanding of insulin signalling, revealing its intricate regulation and links to disease, particularly cardiometabolic disease. Major insights, such as the mechanisms of AKT activation and the influence of genetic and environmental factors, have emerged from studying this network. In this Review, we examine the architecture of insulin signalling, focusing on its precise temporal regulation. We highlight AKT’s central role in insulin action and its vast substrate repertoire, which governs diverse cellular functions. Additionally, we explore feedback and crosstalk mechanisms, such as insulin receptor substrate protein signalling, which integrates inputs through phosphorylation at hundreds of distinct sites. Crucially, phosphoproteomics has uncovered complexities in insulin-resistant states, where network rewiring is characterized by disrupted phosphorylation and the emergence of novel sites that are absent in healthy cells. These insights redefine insulin signalling and its dysfunction, highlighting new therapeutic opportunities.
Read full article here
***
June 2025
Cold exposure stimulates cross-tissue metabolic rewiring to fuel glucose-dependent thermogenesis in brown adipose tissue
This study explored how tissues communicate their metabolic needs during cold exposure, uncovering a new mechanism for glucose-dependent heat production in brown adipose tissue. Strikingly, this pathway was underpinned by coordinated metabolic reprogramming in white adipose tissue and the liver, demonstrating the importance of considering metabolic adaptations in the context of the whole body. Findings also revealed unexpected convergence between the mechanisms of cold adaptation and obesity in brown adipose tissue, suggesting potential new targets for therapeutic intervention for cardiometabolic diseases.
April 2025
Analysis of multiple insulin actions in single muscle fibres from insulin resistant mice reveals selective defect in endogenous GLUT4 translocation
Accurate measurement of GLUT4 translocation is crucial for understanding insulin resistance in skeletal muscle, a key factor in the development of metabolic diseases. However, current methods rely on overexpressed epitope-tagged GLUT4 constructs or indirect measurements, limiting their physiological relevance and applicability. To overcome these challenges, we developed an innovative high-sensitivity imaging-based method that enables the direct assessment of endogenous GLUT4 translocation in primary skeletal muscle fibres. This approach utilises antibodies targeting exofacial epitopes on native GLUT4. Our method allows multiplexed analysis of multiple insulin-sensitive processes, including transferrin receptor trafficking and FOXO nuclear exclusion, alongside mitochondrial oxidative stress. This comprehensive approach provides a unique opportunity to simultaneously assess insulin action across different signalling branches within individual muscle fibres. We validated this method across multiple inbred mouse strains and models of insulin resistance, including chronic insulin exposure, palmitate treatment, and high-fat diet-induced obesity. Notably, we identified a selective defect in GLUT4 trafficking in insulin-resistant muscle fibres, while other insulin-dependent processes remained intact. By offering a high-fidelity model that maintains physiological relevance, this novel approach represents a significant advancement in the study of skeletal muscle insulin resistance and provides a powerful tool for dissecting gene-environment interactions that underlpin metabolic disease.
Judge, S., Masson, S.W.C., Madsen, S., Potter, M., James, D.E., Burchfield, J.G., Diaz-Vegas, A.
October 2024
Meet the Solutionists, with Mark Scott
In your DNA - the future of medicine
Season 3, Episode 4
There's no one else on the planet just like you. So why do you take the same medicine as everyone else when you get sick? Professor David James sees a better way... And it starts with your genes.
Listen to the full podcast here
July 2024
The metabolic consequences of ‘yo-yo’ dieting are markedly influenced by genetic diversity
Weight loss is a powerful tool to improve metabolic health but is almost always followed by weight regain, a term called ‘yo-yo dieting’. Yo-yo dieting is detrimental to metabolism; however, the effect of genetics on yo-yo dieting and whether any protective benefits persist despite weight regain have not been documented before. Our study address this and finds that genetics dictates susceptibility to weight loss and regain. We further show that conclusions in one inbred mouse strain cannot be translated to broader genetic backgrounds.
We are very proud of the paper and this work. It is the brainchild of our dear friend Senthil, whose untimely and tragic passing still moves us. It is also a testimony to great teamwork getting this work published. Congratulation to Aaron for his first ‘first-author’ paper. This would not have happened without you.
Thillainadesan S, Lambert A, Cooke KC, Stöckli J, Yau B, Masson SWC, Howell A, Potter M, Fuller O, Jiang B, Kebede MA, Morahan G, James DE, Madsen S, Hocking S
Read full article here
June 2024
Deletion of miPEP in adipocytes protects against obesity and insulin resistance by boosting muscle metabolism.
Mitochondria facilitate thousands of biochemical reactions, covering a broad spectrum of anabolic and catabolic processes. Here we demonstrate that the adipocyte mitochondrial proteome is markedly altered across multiple models of insulin resistance and reveal a consistent decrease in the level of the mitochondrial processing peptidase miPEP.
Alexis Diaz-Vegas, Kristen C. Cooke, Harry B. Cutler, Belinda Yau, Stewart W.C. Masson, Dylan Harney, Oliver K. Fuller, Meg Potter, Søren Madsen, Niamh R. Craw, Yiju Zhang, Cesar L. Moreno, Melkam Kebede, G. Gregory Neely, Jacqueline Stöckli, James G. Burchfield, David E. James.
Read full article
May 2024
Congratulations to Alexis on winning the 2024 Biomolecular Horizons Young Science Program Award.
Young Scientist Program 2024 (YSP2024) for early career researchers.
YSP2024 will bring together outstanding PhD candidates and early career researchers (<10 years post-PhD) from the FAOBMB region and IUBMB member countries for a 2-day scientific and networking program, prior to the Biomolecular Horizons 2024: Discover, Create, Innovate Congress.
During the 2-day scientific and networking program, the outstanding young scientists will have the opportunity to disseminate their research to an international audience, build their global network with peers, and promote their scientific career. As well as presentations from all participants, YSP2024 will include extensive networking opportunities and career-focused workshops. All participants will receive a Travel Fellowship to support their participation at both YSP2024 and Biomolecular Horizons 2024: Discover, Create, Innovate.
March 2024
In the newest episode of DiabetesBio, the American Diabetes Association’s podcast, hosts Drs. Kirk Habegger and Darleen Sandoval dive into a fascinating discussion with Harry and David, first and last authors of “Dual Tracer Test to Measure Tissue-Specific Insulin Action in Individual Mice Identifies In Vivo Insulin Resistance Without Fasting Hyperinsulinemia.”
They talk about their groundbreaking study on insulin resistance in mice, more specifically the use of the Dual Tracer Test and describe insights into tissue-specific insulin action. They also raise an interesting question: Could it be that the popular belief that peripheral insulin resistance leads to hyperinsulinemia is inaccurate?
The Dual Tracer Test has quickly become a routine component of the lab’s metabolic phenotyping armamentarium and we encourage any others interested in the technique to reach out, we’d love to hear from you.
Tune in to the episode on Apple Podcasts or Spotify, and access the full article for free.
Listen on Apple Podcasts
Listen on Spotify
Read the full article
We would like to congratulate Aaron Lambert and welcome back Billy Jiang to the James Lab as our new PhD candidates.
Aaron has now progressed from accoladed Honours student to PhD candidate as part of the James Lab. His past contributions to the James Lab have ranged from analysing the genetic mechanisms driving metabolic disease to investigating the effects of weight loss and 'yo-yo dieting' on metabolic health.
Aaron is currently undertaking his PhD candidature under the supervision of Professor David James and Dr Soren Madsen, where he is leveraging novel multi-omics and machine learning approaches in adipose tissue to investigate the structural and proteomic landscapes in which disease arises.
Billy just completed the combined Bachelor of Advanced Computing and Bachelor of Science (Medical Science) at the University of Sydney. During the journey, he also completed his Honours study with a First Class Honours in Information Systems at the Faculty of Engineering, where he conducted evaluation studies on NSW’s newly implemented Electronic Medication Management System (eMeds).
By chance, Billy enrolled in a Dalyell unit with the James lab, where he began analysing proteomics datasets and discovered a passion for biological research in metabolic health. He then continued to stay as a Charles Perkins Centre Summer Research Scholar, and got his hand in learning Systems Biology approaches. Recently, he decided to pursue a PhD here, with an interest in understanding the underlying mechanism of metabolically healthy obesity. In particular, he wishes to learn and apply Systems Biology approaches to identify key and novel regulators of adipose tissue glucose metabolism.
September 2023
Congratulations to Alexis on winning the 2023 Ben Barres Spotlight Awards runners up prize of $ 3,500
Ben Barres Spotlight Awards: Announcing the winners for 2023
In a celebration of our diverse researcher community, today we are thrilled to announce the winners and runners-up of the 2023 Ben Barres Spotlight Awards. This annual accolade, now in its fifth year, shines a spotlight on pioneering researchers from groups that are underrepresented in biology and medicine or from countries with limited research funding.
In July and August, for the third consecutive year, the awards also welcomed applications from authors of preprints that have been reviewed publicly by eLife or other reviewing groups on the Sciety platform. This year also saw neurodivergence included among the eligibility criteria for the awards for the first time. In total, 13% of eligible applications received were based on Reviewed Preprints (up from 10% last year), while neurodivergent researchers made up 8% of qualifying applicants.
Dual Tracer Test to measure tissue-specific insulin action in individual mice identifies in vivo insulin resistance without fasting hyperinsulinemia
The ability of metabolically active tissues to increase glucose uptake in response to insulin is critical to whole-body glucose homeostasis. This report describes the Dual Tracer Test, a robust method involving sequential retro-orbital injection of 14C-2-deoxyglucose (14C-2DG) alone, followed 40 min later by injection of 3H-2DG with a maximal dose of insulin to quantify both basal and insulin-stimulated 2DG uptake in the same mouse. The collection of both basal and insulin-stimulated measures from a single animal is imperative for generating high-quality data since differences in insulin action may be misinterpreted mechanistically if basal glucose uptake is not accounted for. The approach was validated in a classic diet-induced model of insulin resistance and a novel transgenic mouse with reduced GLUT4 expression that, despite ubiquitous peripheral insulin resistance, did not exhibit fasting hyperinsulinemia. This suggests that reduced insulin-stimulated glucose disposal is not a primary contributor to chronic hyperinsulinemia. The Dual Tracer Test offers a technically simple assay that enables the study of insulin action in many tissues simultaneously. By administering two tracers and accounting for both basal and insulin-stimulated glucose transport, this assay halves the required sample size for studies in inbred mice and demonstrates increased statistical power to detect insulin resistance, relative to other established approaches using a single tracer. The Dual Tracer Test is a valuable addition to the metabolic phenotyping toolbox.
Cutler, H.B., Madsen, S.,Masson, S.W.C.,Cooke, K.C., Potter. M., Burchfield, J.B.,Stöckli, J., Nelson, M.E., Cooney, G.J., James, D.E.
August 2023
Welcome Oli!
We would like to welcome our newest member to the James lab, Oliver Fuller who has joined us as a post doctoral researcher.
Oli earned his PhD in the Cellular and Molecular Metabolism Lab at the Monash Institute of Pharmaceutical Sciences under the guidance of Professor Mark Febbraio. His research focused on the protective impact of exercise against Alzheimer's Disease (AD) and Non-alcoholic Steatohepatitis (NASH), with a particular interest in the role of exercise-induced Extracellular Vesicles (EVs).
Before this, Oli completed a Master's in Medical Physics at the University of Sydney. There, he researched deep-learning approaches to improve the detection precision of transient changes in neurotransmitter levels during dynamic PET scans.
Oli will be involved in various projects which will include employing deep-learning techniques for enhanced phenotypic classification and exploring the influence of ketone metabolism on insulin resistance.
July 2023
Leveraging genetic diversity to identify small molecules that reverse mouse skeletal muscle insulin resistance
Systems genetics has begun to tackle the complexity of insulin resistance by capitalising on computational advances to study high-diversity populations. ‘Diversity Outbred in Australia (DOz)’ is a population of genetically unique mice with profound metabolic heterogeneity. We leveraged this variance to explore skeletal muscle’s contribution to whole-body insulin action through metabolic phenotyping and skeletal muscle proteomics of 215 DOz mice. Linear modelling identified 553 proteins that associated with whole-body insulin sensitivity (Matsuda Index) including regulators of endocytosis and muscle proteostasis. To enrich for causality, we refined this network by focusing on negatively associated, genetically regulated proteins, resulting in a 76-protein fingerprint of insulin resistance. We sought to perturb this network and restore insulin action with small molecules by integrating the Broad Institute Connectivity Map platform and in vitro assays of insulin action using the Prestwick chemical library. These complementary approaches identified the antibiotic thiostrepton as an insulin resistance reversal agent. Subsequent validation in ex vivo insulin-resistant mouse muscle and palmitate-induced insulin-resistant myotubes demonstrated potent insulin action restoration, potentially via upregulation of glycolysis. This work demonstrates the value of a drug-centric framework to validate systems-level analysis by identifying potential therapeutics for insulin resistance.
Masson, S.W. C., Madsen, S., Cooke, K.C., Potter, M., Diaz Vegas, A., Carroll, L., Thillainadesan, S., Cutler, H.B., Walder, K.R., Cooney, G.J., Morahan, G., Stöckli, J., James, D.E.
June 2023
Farewell Julian
It is a mix of both sadness and excitement that we say farewell to Julian Van Gerwen, who is pursuing a PhD at ETH in Zurich, Switzerland. Julian has been an invaluable member of our lab since joining us in 2019 as a first-year maths and biochemistry undergraduate. Julian worked on analysing omics data generated by the lab to predict mechanisms of metabolic disease, with a focus on leveraging phosphoproteomics to untangle signalling defects underlying insulin resistance. As an honours student, Julian’s work focused on generating phosphoproteomics data to understand how genetic background and diet interact to modulate insulin signalling and insulin resistance.
Julian is a talented and brillant individual and we are excited to see where his journary takes him as we know he will go on to achieve great things.
We wish Julian every success in his future endeavors.
May 2023
In Memory of Senthil Thillainadesan
Senthil Thillainadesan was trained as an Endocrinologist at John Hunter Hospital in Newcastle and Royal Prince Alfred Hospital in Sydney where he developed a deep interest in metabolic disease. Senthil recognised the contribution of basic scientific research to the understanding of metabolic disease and commenced his PhD studies in his final year of endocrinology training at the University of Sydney’s Charles Perkins Centre under the mentorship of Associate Professor Sam Hocking and Professor David James.
Senthil’s project was on the metabolic impacts of weight cycling in mice. From his interactions with patients in the clinic, Senthil was well aware of how difficult it is for people to not just lose weight but to retain weight loss. It had been suggested that periods of weight gain followed by weight loss and so forth – something referred to by Senthil as yo-yo dieting – could be ill-advised for long term health but the evidence to support this was incomplete. Senthil tackled this important question and made the astute observation that animals that underwent repeated bouts of weight gain and loss displayed chronic hyperinsulinemia giving him the first clue that weight cycling could be detrimental to health. Intriguingly, he explored this even further in a population of genetically diverse mice and found that there was incredible variability in the “yo-yo effect” between animals of distinct genetic makeup. During this time Senthil became an expert in genetic analysis and coding and he was the ‘go-to’ person in our lab for these methods. Senthil was generous with his skills and shared everything with anybody. He was a role model for all and a friend to many.
Senthil graduated in April this year as shown below and was incredibly proud to be awarded his PhD. Sadly, he passed away on Sunday May 14, 2023. He will be dearly missed.
February 2023
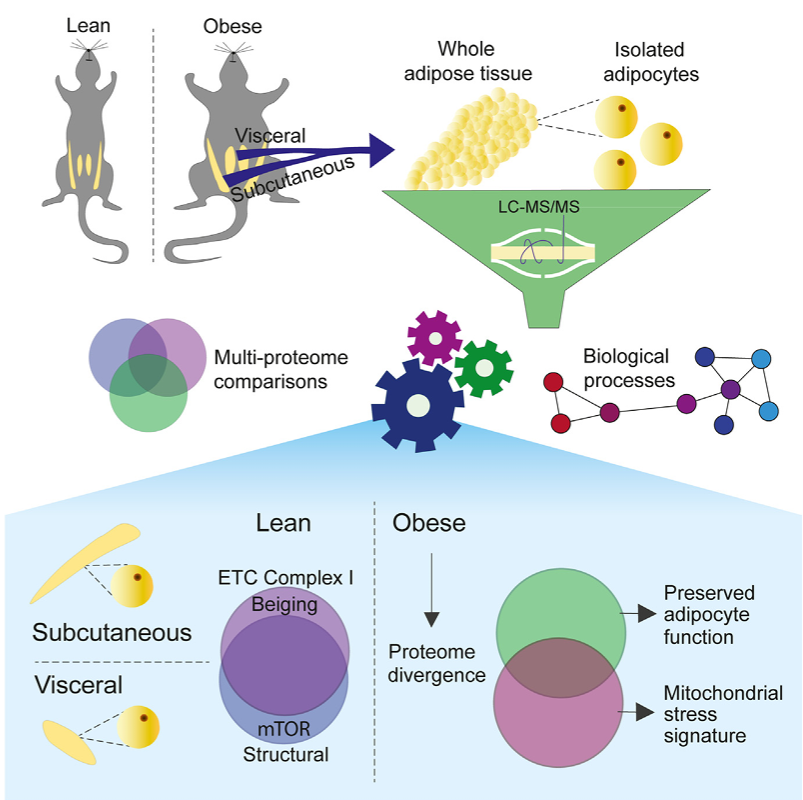
Madsen et.al measured deep proteomes of visceral and subcutaneous adipose tissue and isolated adipocytes in a lean and obesogenic state. In the lean state, the adipocyte proteomes from distinct depots were remarkably similar. A sustained obesogenic diet caused the proteomes to diverge, driven by the visceral adipocytes which presented a maladaptive mitochondrial stress signature. Comparing dietary changes in tissue and adipocytes revealed that changes to the subcutaneous adipocyte proteome could not explain whole tissue changes with in the depot.
January 2023
Congratulations to Soren Madsen and Stewart Masson with their recent success in being awarded Diabetes Australia Research Program grants.
The Diabetes Australian Research Program supports and develops outstanding diabetes research in Australia by funding a range of grants across the full spectrum of diabetes research through a merit based, competitive, peer review process. Research projects can focus on prevention, management of diabetes or the cure for diabetes. Well done Soren and Stewart!
Cross Species Analysis of Hepatic Steatosis - Soren Madsen
Non-alcoholic fatty liver disease (NAFLD) is one of the major health problems in the western world. Despite this, there is currently no approved pharmacological treatment for NAFLD. Both obesity and high fasting insulin and high liver triglycerides are strong risk factors for NAFLD. Here, we utilize the Diversity Outbred Australia (DOz) mice together with the wealth of data from human genetics to find novel drivers of liver fat accumulation. We detect ~100-fold difference in liver triglycerides levels across 243 male DOz mice fed a healthy chow or a high-fat diet concomitant with a 40-fold difference in fasting blood insulin levels, highlighting the DOz population as an excellent model for studying NAFLD. We quantified ~4000 proteins across all livers by total proteomics. By combining the mixed linear modelling and network analysis, overlaid with genes that increase risk of developing NAFLD in the human population, two modules of co-regulated proteins were identified, which were highly associated with liver triglycerides in the mouse livers. Encouraging, proteins involved in DNL, such as SCD1, ACC1, where its inhibitor is currently in clinical trials, were among the proteins in these two modules and interestingly were significantly affect by diet through linear model. By filtering proteins based on features from the linear modelling, such as effect size and significantly affected by triglycerides, we identified 30 proteins, put forward for validation for their role in liver fat accumulation. Currently, we are overexpressing these targets in vitro and assing fat accumulation by microscopy. We are grateful for Diabetes Australia supporting this important work.
A genetic α-defensin variant provides mechanistic insight into the host-microbiome interaction that controls whole body insulin sensitivity - Stewart Masson
Alpha-defensins are a group of antibacterial peptides secreted by specialised cells in the small intestine to modulate microbial composition. There are strong links between the microbiome and host metabolic health and genetic mapping of insulin sensitivity in our DOz mice revealed a QTL centred over the defensin locus. Single nucleotide polymorphism (SNP) analysis of our mice revealed that mice harbouring the rs238754102 SNP with this locus exhibited 50 % lower insulin levels during a glucose tolerance test, and reduced liver triglycerides. By utilising metabolomics and multi-strain mouse studies we hope to untangle how defensin genetics contributes to microbiome composition and host metabolic health. We’d like to thank Diabetes Australia for supporting this work
December 2022
Big Congratulations to all of the following lab members who have recently achieved great things!
Dr Sean Humphrey, Postdoctoral Research Fellow has moved to Murdoch Institute in Melbourne as a Lab Head. Congratulations, Sean!
Dr Senthil Thillainadesan was awarded his PhD. Senthil has decided to return to clinical medicine. We will miss you, Senthil!
Dr Sophie Trefely (ex-student) has secured a position at Cambridge University. This makes two from our lab with Dr Daniel Fazakerley having been there for almost 4 years as a new Lab Head. What a fantastic achievement both.
Professor James attended the Australian Laureate Fellowships Pin Ceremony held at the Parliament House in Canberra. Australian Laureate Fellows were awarded with a special Pin recognising their work with the international research community
January 2022
The Garden of Diverse Genetics.
Muscle and adipose tissue make up different paths. The BL/6 mouse looks longingly for other strains to be included in metabolic research. Whatever it is, the way you tell your story online can make all the difference.
This latest research is a stepping stone for our group to continue to paint a fascinating story of metabolism.
Artwork by Marin Nelson
*review the full article below*
December 2021
First Class Honours
It is a great pleasure that our two young understudies, Harry Cutler and Harrison Hoffman, have been informed that they received first class Honours with an additional award of the University Medal. This is outstanding achievement for both of these young men who did a magnificent job this year. I think both of these guys became a solid part of the family and we accepted into the fold. Also, it is important to add that many people provided both of them with tremendous help and support and this is incredibly collegial and generous of those people. Thank everybody for playing a part.
We wish Harry and Harrison a very Merry Celebration and we look forward to seeing Harry next year and Harrison at some point in the future when we require intricate surgical procedures hopefully at mates rates.
Congratulations !
Congratulations Elise we will miss you!
PhD student Elise Needham has just been informed she has received an EMBO Postdoc fellowship to go to Cambridge University to support the next phase of her career. Elise has done an amazing job in our lab both acquiring and analysing phospho data capitulating recently in a first author Nature Biotech paper from her work on exercise with our Copenhagen friends.
Revolutionising our understanding of type 2 diabetes in diverse populations
A joint research project co-led by Professor David James and Dr Sean Humphrey from the University of Sydney and Professor Jørgen Wojtaszewski from the University of Copenhagen, could revolutionise our understanding of type 2 diabetes in diverse populations and provide new drug targets, initiating the development of Precision Medicine strategies to tailor appropriate treatments.
Exercise is known to have profoundly beneficial effects for people with diabetes as it improves insulin sensitivity. However, the mechanisms for this effect have eluded researchers for decades. By teaming up with researchers in Copenhagen, world leaders in exercise research in humans, James and Humphrey have applied their cutting edge mass spectrometry methods to study protein phosphorylation in people following exercise. One of the striking findings to emerge from this work is that each individual was found to possess a unique pattern of protein phosphorylation spanning many thousands of data points. By utilising these unique patterns they were able to for the first time begin to discover novel aspects of the molecular circuitry used by exercise to encode its beneficial effects.
Farewell Guang
Guang Yang a member of our team for 11 years moves to UNSW to work with Rob Yang. Guang has been like a brother to many of us and we are really sad to see him leave. But in science it is crucial to move around and continually enrich your horizons and Guang felt he needed to take this new step. Guang published a number of pivotal studies when he was in the lab and I am sure he will continue to make amazing contributions to science.
Good luck Guang and congratulations!
November 2021
Congratulations to James Burchfield and Sean Humphrey with their recent success in being awarded NHMRC Ideas grants. The success rate for this scheme is under 10%, which highlights the exceptional quality of work being undertaken by these researchers. Well done James and Sean! Thanks also to everybody that contributed to these grants – so many people gave up their time and this is so much appreciated.
Dr James Burchfield has been awarded $1.7 million to better understand the underlying cause of Coenzyme Q10 (a molecule essential for energy production) depletion and how it causes cardiometabolic disease. The aim is to use newly acquired understanding of the disease process to develop new treatments.
Dr Sean Humphrey has been awarded $1.3 million for Mapping the Beneficial Effects of Exercise in humans using Personalised Phosphoproteomics.
October 2021
New insights into mTORC1 biology
Guang Yang, a senior postdoc in the group, has published a study in Science Signaling detailing the features of next gen TOR inhibitors that provide very novel insights both into TOR Biology and rapamycin biology.
Read full article *here*
July 2021
Scientists help solve insulin puzzle
Development could enhance treatments for diabetes and cancer
Researchers have identified a cell communication pathway that controls cell growth and survival, and the finding could eventually help to develop treatments for diseases such as cancer and diabetes.
Effective communication, crucial to human relationships, is also essential for the destruction of cancer cells within the body.
In the body’s cells, communication involves the transmission of molecular or chemical signals. Just as a faulty antenna results in a garbled TV image, if these molecular signals are distorted, information is lost, and the outcomes can be catastrophic.
Researchers from the Charles Perkins Centre, University of Sydney and Monash University’s Biomedicine Discovery Institute have recently identified a cell communication pathway that controls cell growth and survival. The finding could eventually help to develop treatments for diseases such as cancer and diabetes.
Central to the discovery was a powerful combination of cell biology (conducted by PhD students Alison Kearney and Dr Dougall Norris under the supervision of Dr James Burchfield and Professor David James, University of Sydney) and mathematical modelling (conducted by Milad Ghomlaghi under the supervision of Dr Lan Nguyen, Monash University).
“We were able to see the molecules involved in communication, and therefore, see when and where they are needed in the cell, and what happens when things go wrong,” Dr Burchfield said.
Dr Nguyen described the discovery as a "powerful regulator of the insulin signalling network”.
Read the paper *here*
Comprehensive Review about Insulin Resistance
David and Jacky teamed up with Morrie Birnbaum to write this extensive review for Nature Reviews Mol Cell Bio
Read full article *here*
The aetiology and molecular landscape of insulin resistance
David E. James, Jacqueline Stöckli & Morris J. Birnbaum
https://doi.org/10.1038/s41580-021-00390-6
March 2021
Australian team gets to the heart of COVID-19
Sydney’s mass spectrometry capabilities advance COVID-19 trials
A collaboration between Professor David James and Associate Professor James Hudson from QIMR Berghofer have uncovered some of the ways COVID-19 affects the heart and identified potential drug treatments which have progressed to clinical trials.
The project tackled one of the major complications of COVID-19 – the “cytokine storm.” This occurs when the immune system overreacts to the infection, releasing inflammatory molecules called ‘cytokines’ into the bloodstream.
The research was recently published in Cell.
Read Full article *here*
February 2021
We would like to welcome our newest member to the James lab Stewart Masson who has joined us as a post doctoral researcher.
Stewart has just moved from New Zealand and completed his PhD in the Department of Nutrition at the University of Auckland’s under supervision of Troy Merry and Peter Shepherd. During Stewart’s PhD he investigated the structural protein β-catenin as a potential regulator of actin remodelling during GLUT4 translocation. Prior to his PhD, Stewart completed a master’s degree in the School of Biological Sciences, also at the University of Auckland, where he investigated mitochondrial thermogenesis in insect flight muscle.
Stewart will be investigating the role of genetics in metabolic disease and healthy aging and his interests lie in the aetiology of insulin resistance, and the mechanics of intracellular trafficking, particularly how it relates to metabolism.
January 2021
ARC LIEF Outcomes
The Australian Research Council (ARC) Linkage Infrastructure, Equipment and Facilities (LIEF) application lead by Professor James and Dr Burchfield for an “Integrated Multimodal System for Multiplexed Imaging of Signal Transduction” was successful in being awarded $900,000.
This project will introduce a unique microscopy platform and associated technologies into the Australian research environment that will enable researchers to redefine our understanding of molecular signal transduction. The instrumentation will enable the multidimensional imaging of live cells with unprecedented speed and sensitivity. Read more here.
October 2020
Universities caught between COVID and funding cuts
Professor David James’ and PhD student Elise Needham appear on ABC’s 7:30 Report about the COVID recession and how it has impacted the higher education.
September 2020
After a meal, the hormone insulin promotes the production of fat as a means of storing energy. Fat can be made from a range of nutrients, such as carbohydrates and proteins. Here we found that insulin specifically requires glucose (sugar) to make fat. Using a range of techniques to study metabolism, including cutting-edge metabolomics technologies, we demonstrated that glucose provided the carbon and energy required for fat production. Glucose was also needed by insulin to turn off fat burning and conserve amino acids for energy and protein synthesis – these are examples of one nutrient controlling the metabolism of other nutrients. Overall, glucose was needed for fat production, which we found to be essential in fat cell development and for the ability of animals to survive starvation. These studies demonstrate that just as insulin acts to reduce blood glucose levels, conversely insulin needs glucose to mediate its effects on promoting energy storage.
July 2020
Professor David James has been awarded a 2020 Australian Research Council (ARC) Laureate Fellowship.
Professor James has been awarded a prestigious Australian Laureate Fellowship from the ARC.
Professor David James has been awarded a prestigious Australian Laureate Fellowship from the ARC.
The Fellowship will enable Professor James to continue research which aims to expand our current understanding of aging and provide new therapeutic options for improving healthy aging.
“This is the first time in my career that I feel I have been able to tackle and commit to such a highly ambitious and far reaching project,” said Professor James.
“Healthy aging is determined by a complex interaction between our genetics and the environment in which we live. If we are to understand the aging process it is critical that we begin to decipher this complex interaction,” explained Professor James.
“This is very difficult to achieve in humans largely because of the challenge in both quantifying and controlling the environment.
"To overcome this challenge we have designed a very exciting approach employing a highly unique mouse population that mirrors the genetic diversity found in the human population,” said Professor James.
By exposing the mouse population to unique environments and studying health outcomes, Professor James believes he will be able to better navigate the gene-environment interaction landscape that underpins the aging process and expand our understanding of aging.
Funding to support research and nurture early-career researchers
Awarded to up to 17 researchers each year, the ARC Laureate Fellowship supports ground-breaking, internationally-competitive basic and applied research by providing project funding, a salary supplement and funding for postdoctoral and postgraduate researchers.
“In addition to helping continue my research, this fellowship will provide me with the capacity to attract and facilitate the careers of young researchers of outstanding quality at the University of Sydney,” said Professor James.
“While this scheme is an important vehicle to enable researchers to tackle critical challenges facing our planet, we need to continue investing in basic research without any boundaries to enable the talented pool of researchers that Australia has at its fingertips to develop their careers.”
When asked what advice he would give to peers thinking about applying for the ARC Laureate Fellowship scheme, Professor James said his advice was simple.
“Be bold, be big and be clear.”
April 2020
Research into COVID-19
One of the major pathological features of COVID-19 infection is organ failure, particularly heart failure. This is often triggered by what is referred to as a “cytokine storm”, a massive immune response that mysteriously causes organ damage and ultimately death. Prof David James, Dr Sean Humphrey and PhD student Elise Needham from the Charles Perkins Centre have teamed up with A/Prof James Hudson and colleagues of QIMR Berghofer in QLD to try to identify druggable pathways triggered by cytokine storm syndrome. To do this, they will use a unique cardiac organoid model developed by Hudson, combined with advanced phosphoproteomics analysis at Sydney Mass Spectrometry.
The project has gone from a funding call to funded in a matter of weeks, with the first experiments already underway. The samples for analysis should be arriving by early May. If this research were to identify a drug that could dampen the impact of the cytokine storm on organs like the heart this could have a huge impact on the COVID-19 death rate in the human population, as well as a multitude of other conditions that frequently invoke this immune response, such as CAR-T therapy, treatment with certain life-saving drugs, as well as other severe infections.
December 2019
Congratulation to PhD student - Elise Needham who was awarded the Best Student Presentation for the 2019 Biology Domain Seminar Series in the Charles Perkins Centre!
November 2019
Professor David James was awarded an honorary doctorate from the University of Copenhagen for his outstanding contribution to research in physiology, biochemistry and metabolism.
The honorary doctorate is awarded at the Annual Commemoration at the University of Copenhagen.
About the Annual Commemoration
The University of Copenhagen was founded in 1479. Ever since, the University has celebrated its foundation with an annual commemoration. The commemoration is always held on the third Friday in November with participation from the Royal Family.
The commemoration is composed of a Ceremony in the Ceremonial Hall of the University at Frue Plads in the afternoon and an external evening event.
The University management invites more than 700 internal and external guests for the Commemoration Ceremony. These are representatives of the University's employees and students, of research councils, funds, ministries and other close partners as well as the University's different prize winners, appointed doctors and honorary doctors of the year.
Full article - Distinguished physiologist honorary doctor at the University of Copenhagen
November 2019
The challenge of understanding complex physiological processes lies at the heart of modern medicine. These include questions like, “how does exercise promote health?” and, “why are some drugs more effective on some individuals?”. We devised a strategy to dissect complex processes, in this case exercise, in the hopes of better understanding some of the mechanisms underpinning it. To achieve this, we selected a range of clinically approved drugs, as well as broad stimulants involved in exercise, such as heat stress. Detailed measures of cellular behaviour were taken with cutting edge mass spectrometry-based technologies. Measuring the effect of different treatments enabled us to segment and study the different components that make up the complex physiological response to exercise. Studying the actions of these molecules on the cell taught us some surprising effects. For example, Aripiprazole is a commonly prescribed medicine used to treat schizophrenia. One side-effect of this drug is high blood sugar, which has implications for diabetic patients. We found that Aripiprazole blocks the molecular transporter that brings sugar from the blood into cells, revealing why this side effect may occur. Since exercise involves multiple stimuli working together, we also explored the effect of combining different treatments on cells. Surprisingly, combining treatments together had vastly different effects compared to the individual treatments, revealing interactions that may occur in exercise. This finding strongly emphasises the need to better understand how drugs interact on a cellular level. This is critical given that many people are prescribed multiple drugs, despite limited knowledge about how they impact the body when they are combined. Performing detailed measures on a panel of treatments has mapped the complex biology of exercise and revealed surprising insights about common medicines.
June 2019
Protein phosphorylation is a major regulator of transcriptional and/or epigenetic control during early embryogenesis. Yet the timing, extent and composition of molecular events, including phosphorylation, that underpin the developmental transformation of pre-implantation epiblast to post-implantation epiblast remain poorly understood. In collaboration with the National Institutes of Health and the Max Planck Institute of Biochemistry we have performed extensive mapping of global changes to the proteome, phosphoproteome, transcriptome, and epigenome of embryonic stem cells transitioning from naïve to primed pluripotency, uncovering a complex and multi-layered network controlling pluripotency progression.
This work is published in the Journal Cell Systems, and the data can be explored at www.stemcellatlas.org
Citation:
Yang P*, Humphrey SJ*, Cinghu S*,… James DE, Mann M and Jothi R. Multi-Omic Profiling Reveals Dynamics of the Phased Progression of Pluripotency. Cell Systems 8, 1-19, (2019).
February 2019
Early signs of fatty liver disease detected in blood
Accumulation of fat in the liver, known as fatty liver disease, is experienced by over 5.5 million Australians, including more than 40 percent of all adults over the age of 50.
Fatty liver develops from a combination of both genetic and environmental causes, which influence the age of onset and severity of the disease. Experts are now describing the condition as a hidden epidemic, which is driving up rates of liver transplant, contributing to a range of illnesses and ultimately death.
Fatty liver disease usually has no early symptoms and diagnoses with current technologies mostly comes too late to prevent major illness.
But now, for the first time in a study published in the prestigious scientific journal, Nature, a team of researchers from the Baker Heart and Diabetes Institute, University of California and University of Sydney, have discovered biomarkers in the blood that can predict the accumulation of toxic fats in the liver, which are a sign of early fatty liver disease. These predictions can be made based on the lipid (fats) profile in the blood.
Dr Benjamin Parker, who was an NHMRC Research Fellow at the Charles Perkins Centre and the School of Life and Environmental Sciences at the University of Sydney and drove the project, said the study aimed to understand the underlying mechanisms of metabolic diseases such as diabetes.
“Some individuals are more susceptible than others and we don't completely understand why,” Dr Parker said.
“Our study used a new approach to link mutations in DNA to changes in fat metabolism. We have identified several new drug targets and biomarkers to treat or monitor fatty liver disease which is strongly associated with diabetes."
Professor David James from the University of Sydney’s Charles Perkins Centre described the study as a technological tour de force that represented one of the most futuristic approaches for examining complex diseases like diabetes and cardiovascular disease.
“We have simultaneously measured many different layers of the biological system including lipids and other metabolites as well as thousands of proteins and integrated this with genetic information,” he said.
“This has given us an exciting view of how complex diseases like fatty liver occur. Most importantly this approach represents a new way forward in precision medicine, an approach which will transform health care.
The team is now hoping to establish why some people are more prone to fatty liver disease than others.
***
January 2019
A big Congratulations to Elise Needhm and Sean Humphrey along with Tim Burykin from the Life Lab for their review article that was published in Science Signaling. Their paper was accompanied by the Front Cover and a Focus article by the Editor Mike Yaffe from Harvard.
The article describes why Cancer research and research into other complex diseases must shift more toward biochemistry and analysis of signal transduction as this represents the disease coal face.
***
Farewell to two of our lab members - Dr Benjamin Parker and Dr Tim Su!
Ben will be heading to the University of Melbourne where he has been appointed as a group leader. Tim has been appointed as the Assistant Director at ChemPartner Co in Shanghai.
We wish them both all the best for the future. They will be missed around the lab
***
We would like to welcome both Dr Alexis Diaz and Dr Soren Madsen who have both joined the James Lab as a Postdoctoral Research Associates.
Alexis obtained his Ph.D. in Biomedical Science working with Dr. Enrique Jaimovich in the Muscle Physiology Lab, Faculty of Medicine, University of Chile. During this time, Alexis learnt about the adult skeletal muscle physiology and its metabolic alteration in diet-induced insulin resistance.
In 2018 Alexis won a research fellow to work in mitochondrial metabolism in neonatal cardiomyocyte with Dr. Sergio Lavandero, in the Advanced Center of Chronic Diseases, University of Chile. He was also the national head of the human physiology undergraduate course to Medical Doctor, School of Medicine, University Andrés Bello, Chile.
Soren completed his PhD in 2018 at the University of Copenhagen with Sara Vienberg and Jonas Treebak at CBMR Integrative Physiology. During Soren’s PhD research he studied adipose tissue biology in relation to metabolic stress, such as high-fat diet and exercise. More specifically he was interested in how miRNA matures during times of low or high energy states, as it has become apparent that miRNAs are dysregulated in metabolic disease.
Soren’s current project investigates how genes and environment affect the progression of insulin resistance. Soren’s main focus is on adipose tissue and trying to identify genetic drives that impair glucose uptake into fat.
It is great to have you both on board!
***
December 2018
Big Congratulations to all of the following lab members who have achieved some special things recently.
What a great way to wrap up 2018!!
Dr Benjamin Parker has been appointed as a group leader at the University of Melbourne starting in 2019
Dr Daniel Fazakerley has been appointed as a group leader at Cambridge University starting in May 2019
Our PhD student Elise Needham won the best student oral presentation of the year at the Charles Perkins Centre Biology Domain Seminar Series.
Sean Humphrey and his wife are expecting a little baby in January. Go Sean!
James Krycer won the Skip Martin fellowship for 2019.
Daniel Fazakerely received a DART grant.
Well done to Guang Yang on his EMBO J paper on RagC phosphorylation
***
July 2018
Membrane Topology of Trafficking Regulator of GLUT4 1 (TRARG1).
Duan et al. describe the membrane topology of trafficking regulator of GLUT1 (TRARG1; formally known as TUSC5). We previously identified TRARG1 as a regulator of GLUT4 trafficking in 2015 (click here)
Duan X, Krycer JR, Cooke KC, Yang G, James DE, Fazakerley DJ.
***
Muscle and adipose tissue insulin resistance: malady without mechanism?
Fazakerley, Krycer et al. review mechanisms of muscle and adipose insulin resistance.
Fazakerley DJ, Krycer JR, Kearney AL, Hocking SL, James DE.
***
May 2018
Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation.
Our collaboration with Mike Murphy (University of Cambridge) and Richard Hartley (University of Glasgow) using a novel compound to show that mitochondrial oxidative stress rapidly induces insulin resistance independently of changes in mitochondrial respiration.
Fazakerley DJ, Minard AY, Krycer JR, Thomas KC, Stöckli J, Harney DJ, Burchfield JG, Maghzal GJ, Caldwell ST, Hartley RC, Stocker R, Murphy MP, James DE.
***
February 2018
Mitochondrial CoQ deficiency is a common driver of mitochondrial oxidants and insulin resistance.
Fazakerley et al. identify that loss of mitochondrial CoQ is a proximal driver of mitochondrial oxidants and insulin resistance.
See featured on 7News - Click here
See featured in the eLife podcast - Click here
Fazakerley DJ, Chaudhuri R, Yang P, Maghzal GJ, Thomas KC, Krycer JR, Humphrey SJ, Parker BL, Fisher-Wellman KH, Meoli CC, Hoffman NJ, Diskin C, Burchfield JG, Cowley MJ, Kaplan W, Modrusan Z, Kolumam G, Yang JY, Chen DL, Samocha-Bonet D, Greenfield JR, Hoehn KL, Stocker R, James DE.
***
January 2018
A gas trapping method for high-throughput metabolic experiments
Krycer et al. developed a novel gas-trapping method for high-throughput metabolic experiments. Adapted for cells grown in 12- or 24-well plates, this can be used to measure substrate oxidation by collecting CO2 or hydrogen sulphide production from cysteine metabolism.
Krycer JR, Diskin C, Nelson ME, Zeng XY, Fazakerley DJ, James DE
***
The transcriptional response to oxidative stress is part of, but not sufficient for, insulin resistance in adipocytes
It is well-established that reactive oxygen species (ROS) are linked to insulin resistance. Chaudhuri, Krycer et al. found that although ROS induces substantial changes across the transcriptional landscape in adipocytes, this transcriptional response is not required for ROS to cause insulin resistance. This suggests that ROS acts via other ‘omes, such as post-translational modifications, to cause insulin resistance in adipocytes.
Chaudhuri R, Krycer JR, Fazakerley DJ, Fisher-Wellman KH, Su Z, Hoehn KL, Yang JYH, Kuncic Z, Vafaee F, James DE.
***
December 2017
Dynamic Metabolomics Reveals that Insulin Primes the Adipocyte for Glucose Metabolism
Krycer et al. explored how insulin regulates adipocyte metabolism. It is widely-held that energy storage (anabolism) occurs as a substrate accumulates. However, using dynamic tracer metabolomics and overlaying phosphoproteomics data, they found insulin signalling triggers anabolism before substrates accumulated, creating a ‘demand-driven’ system to prime adipocytes for glucose metabolism.
Krycer JR, Yugi K, Hirayama A, Fazakerley DJ, Quek LE, Scalzo R, Ohno S, Hodson MP, Ikeda S, Shoji F, Suzuki K, Domanova W, Parker BL, Nelson ME, Humphrey SJ, Turner N, Hoehn KL, Cooney GJ, Soga T, Kuroda S, James DE
***
Editor's pick at JBC!
Metabolomic analysis of insulin resistance across different mouse strains and diets
"Insulin resistance is a complex condition with many genetic and environmental determinants. Stöckli et al carried out a comprehensive metabolomic analysis of three different mouse strains on high-fat or standard diets. The analysis showed that, despite individual, environmental, and genetic variation, a combination of three metabolites (C22:1-CoA, C2-carnitine, and C16-ceramide) together formed an accurate signature for predicting insulin resistance."
Jacqueline Stöckli, Kelsey H. Fisher-Wellman, Rima Chaudhuri, Xiao-Yi Zeng, Daniel J. Fazakerley, Christopher C. Meoli, Kristen C. Thomas, Nolan J. Hoffman, Salvatore P. Mangiafico, Chrysovalantou E. Xirouchaki, Chieh-Hsin Yang, Olga Ilkayeva, Kari Wong, Gregory J. Cooney, Sofianos Andrikopoulos, Deborah M. Muoio, and David E. James
***
Multiplexed Temporal Quantification of the Exercise-regulated Plasma Peptidome
The latest paper by Parker et al. has been one the most highly viewed article of the Journal of Molecular & Cellular Proteomics during the past month!
Benjamin L. Parker, James G. Burchfield, Daniel Clayton, Thomas A. Geddes, Richard J. Payne, Bente Kiens, Jørgen F. P. Wojtaszewski, Erik A. Richter and David E. James